- Research Interest
- Selected Publications
- Professional Experience
- Research Group
Cell-to-cell variability is an inherent feature of cell populations and multicellular organisms. Such plasticity is essential for embryo development and serves as a mechanism of tissue regeneration but it can also predispose tissues to malignant transformation. How cell heterogeneity emerges during development and contributes to disease is a fundamental question in biology.
In the laboratory we are interested in determining the roles of cell heterogeneity in development and regeneration, and are exploring strategies to unlock phenotypic plasticity in pluripotent and cancer cells.
Common Mechanisms of Cell Plasticity in Development and Cancer
Cell heterogeneity not only arises from the coexistence of different cell subsets, but also from cells alternating between interconvertible metastable states. The main source of such plasticity is the Epithelial-Mesenchymal Transition (EMT) that is not confined to physiological embryonic development but is reactivated at the onset of several pathologies, including tumor progression/metastasis and fibrosis.
In the laboratory we use different strategies based on development-inspired cues and unbiased high-throughput phenotypic screenings to investigate the mechanisms that control phenotypic plasticity in stem cell population and cancer cells. We also use 3D gastruloids and tumour organoids to study how cell heterogeneity influences the self-organization properties of stem cells.
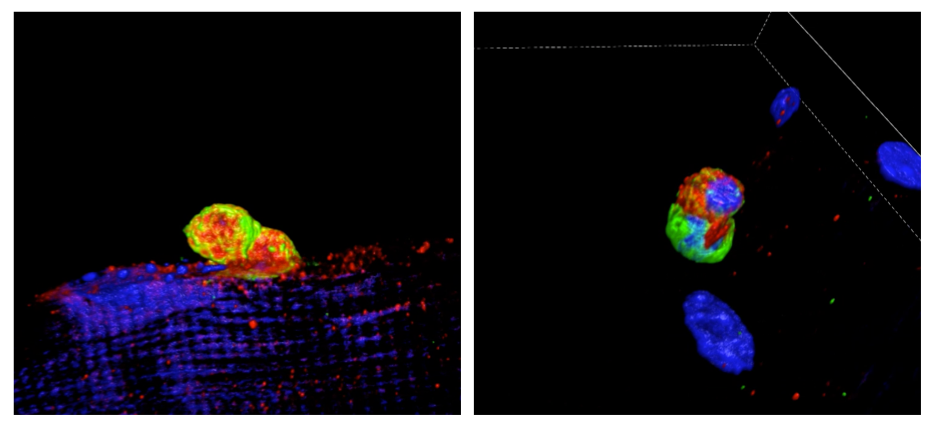
Cell Hetherogeneity in Skeletal Muscle Regeneration
Skeletal muscle has a remarkable regenerative capacity that relies on highly coordinated interactions of different cell populations, including muscle stem cells/satellite cells, macrophages and endothelial progenitors. In chronic muscle disorders and in aging this repair mechanism does not work properly and the damage cannot be resolved, resulting in severe muscle wasting and prolonged functional disability.
We are investigating the role of the TGFß ligand coreceptor Cripto in this complex scenario, and in particular in the cellular and molecular mechanisms that link inflammation and regeneration, which still remain largely unclear. https://renoir-itn.eu/
- Gastruloid Development Competence Discriminates Different States of Pluripotency. Cermola F, D’Aniello C, Tatè R, De Cesare D, Martinez-Arias A, Minchiotti G, Patriarca EJ. Stem Cell Reports. 2021 Feb 9;16(2):354-369.
- The Multifaceted Roles of Proline in Cell Behavior. Patriarca EJ, Cermola F, D’Aniello C, Fico A, Guardiola O, De Cesare D, Minchiotti G. Front Cell Dev Biol. 2021 Aug 12;9:728576.
- Cripto shapes macrophage plasticity and restricts EndMT in injured and diseased skeletal muscle. Iavarone F, Guardiola O, Scagliola A, Andolfi G, Esposito F, Serrano A, Perdiguero E, Brunelli S, Muñoz-Cánoves P, Minchiotti G. EMBO Rep. 2020 Apr 3;21(4):e49075.
- Metabolic-Epigenetic Axis in Pluripotent State Transitions. D’Aniello C, Cermola F, Patriarca EJ,Minchiotti G. 2019 Jul 31;3(3):13.
- Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Fico A, Fiorenzano A, Pascale E, Patriarca EJ,Minchiotti G. Cell Mol Life Sci. 2019 Apr;76(8):1459-1471.
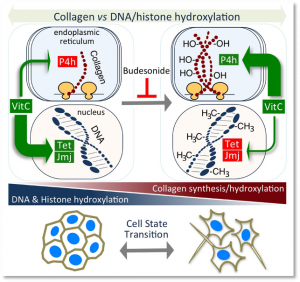
- Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. D’Aniello C, Cermola F, Palamidessi A, Wanderlingh LG, Gagliardi M, Migliaccio A, Varrone F, Casalino L, Matarazzo MR, De Cesare D, Scita G, Patriarca EJ, Minchiotti G. Cancer Res. 2019 Jul 1;79(13):3235-3250.
- An Ultraconserved Element Containing lncRNA Preserves Transcriptional Dynamics and Maintains ESC Self-Renewal. Fiorenzano A, Pascale E, Gagliardi M, Terreri S, Papa M, Andolfi G, Galasso M, Tagliazucchi GM, Taccioli C, Patriarca EJ, Cimmino A, Matarazzo MR,Minchiotti G, Fico A. Stem Cell Reports. 2018 Mar 13;10(3):1102-1114.
- Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. Guardiola O, Andolfi G, Tirone M, Iavarone F, Brunelli S,Minchiotti G. J Vis Exp. 2017 Jan 1;(119):54515.
- Vitamin C and l-Proline Antagonistic Effects Capture Alternative States in the Pluripotency Continuum. D’Aniello C, Habibi E, Cermola F, Paris D, Russo F, Fiorenzano A, Di Napoli G, Melck DJ, Cobellis G, Angelini C, Fico A, Blelloch R, Motta A, Stunnenberg HG, De Cesare D, Patriarca EJ, Minchiotti G. Stem Cell Reports. 2017 Jan 10;8(1):1-10.
- Cripto is essential to capture mouse epiblast stem cell and human embryonic stem cell pluripotency. Fiorenzano A, Pascale E, D’Aniello C, Acampora D, Bassalert C, Russo F, Andolfi G, Biffoni M, Francescangeli F, Zeuner A, Angelini C, Chazaud C, Patriarca EJ, Fico A, Minchiotti G. Nat Commun. 2016 Sep 2;7:12589.
- Conditional Cripto overexpression in satellite cells promotes myogenic commitment and enhances early regeneration. Prezioso C, Iaconis S, Andolfi G, Zentilin L, Iavarone F, Guardiola O,Minchiotti G. Front Cell Dev Biol. 2015 May 21;3:31.
- A novel autoregulatory loop between the Gcn2-Atf4 pathway and (L)-Proline [corrected] metabolism controls stem cell identity. D’Aniello C, Fico A, Casalino L, Guardiola O, Di Napoli G, Cermola F, De Cesare D, Tatè R, Cobellis G, Minchiotti G. Cell Death Differ. 2015 Jul;22(7):1094-105.
- The G-protein-coupled receptor APJ is expressed in the second heart field and regulates Cerberus-Baf60c axis in embryonic stem cell cardiomyogenesis. D’Aniello C, Fiorenzano A, Iaconis S, Liguori GL, Andolfi G, Cobellis G, Fico A,Minchiotti G. Cardiovasc Res. 2013 Oct 1;100(1):95-104.
- L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Comes S, Gagliardi M, Laprano N, Fico A, Cimmino A, Palamidessi A, De Cesare D, De Falco S, Angelini C, Scita G,Patriarca EJ, Matarazzo MR, Minchiotti G. Stem Cell Reports. 2013 Oct 10;1(4):307-21. (COVER PAGE)
- Cripto regulates skeletal muscle regeneration and modulates satellite cell determination by antagonizing myostatin. Guardiola O, Lafuste P, Brunelli S, Iaconis S, Touvier T, Mourikis P, De Bock K, Lonardo E, Andolfi G, Bouché A, Liguori GL, Shen MM, Tajbakhsh S, Cossu G, Carmeliet P,Minchiotti G. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):E3231-40.
- A small synthetic cripto blocking Peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson’s disease. Lonardo E, Parish CL, Ponticelli S, Marasco D, Ribeiro D, Ruvo M, De Falco S, Arenas E,Minchiotti G. Stem Cells. 2010 Aug;28(8):1326-37.
Gabriella Minchiotti has been trained in biology and received her PhD in Molecular and Cellular Genetics in 1997 from the University of Naples Federico II. During her doctoral and post-doctoral studies, she has worked in National and International research Centers, including the European Institute of Oncology (IEO) in Milan, the Ecole Normale Superieure in Paris and the VIB, Center for Transgene Technology & Gene Therapy of Lueven, Belgium, enriching her developmental and cell biology background.
She has run an independent laboratory at IGB-CNR since 2004. She is also co-coordinator of the Stem Cell Fate Laboratory (http://www.igb.cnr.it/scfl/) at IGB-CNR, which started in 2004 with the aim of developing new strategies to improve the use of stem cell therapy for degenerative disorders. Currently she is CNR Research Director at the IGB and Head of the Cell Differentiation Laboratory.
Since 2013 she is member of the Steering Committee of Fondazione AIRC Campania (https://www.airc.it/fondazione/chi-siamo/comitati-regionali/comitato-campania), and she actively collaborates with AIRC’s communication and dissemination activities.
![]() La Ricerca sul Divano | Ep. 5 | Gli organoidi: embrionali ma promettenti |
La Ricerca sul Divano | Ep. 5 | Gli organoidi: embrionali ma promettenti | ![]()
![]()
![]() La possibile seconda vita del budesonide per la cura del tumore al pancreas
La possibile seconda vita del budesonide per la cura del tumore al pancreas
![]() Podcast Fondamentale | Ep. 46 | Tumore al pancreas, una nuova possibile terapia con un anti-asmatico
Podcast Fondamentale | Ep. 46 | Tumore al pancreas, una nuova possibile terapia con un anti-asmatico